|
Meghan Azad, PhD
Scientific Director |
Kelsey Fehr, MSc
Biorepository Technician |
Field Site Partners
|
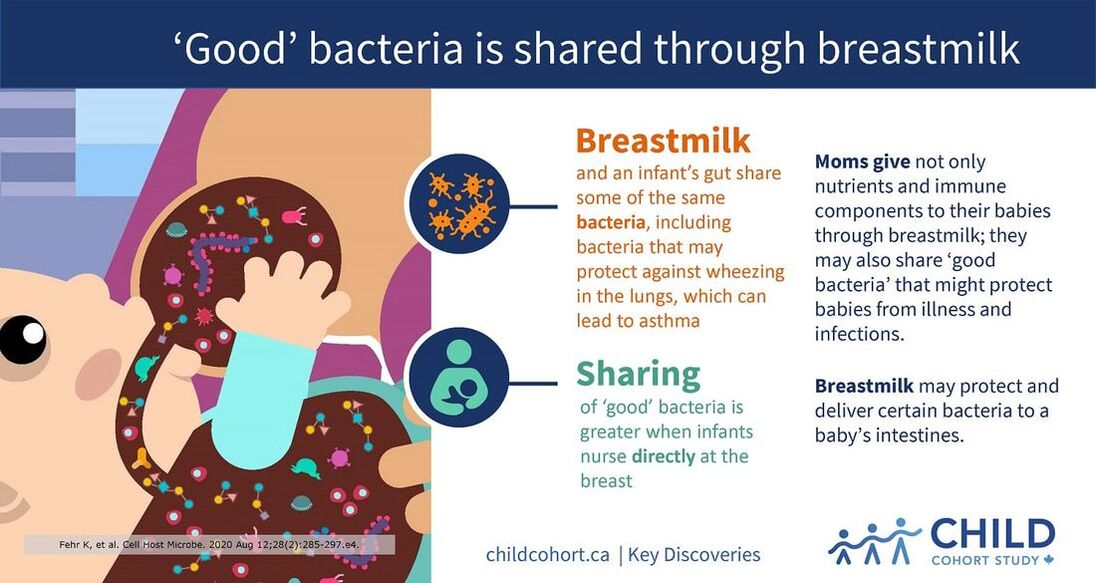
The CHILD Cohort Study is a prospective longitudinal birth cohort study. This means that CHILD researchers are actively following the Study participants over time as they grow and develop—from mid-pregnancy into childhood and adolescence. CHILD is designed this way so it can collect information at time points that are considered to be especially critical to the health and development of children. CHILD contributes milk samples to IMiC for various analyses.
Website: https://childstudy.ca/ |
|
Padmaja Subbarao, MD, MSc, FRCP(C), FAAP, LMCC
Director, CHILD Cohort Study As Study Director, Dr. Subbarao is responsible for the broad scope of conduct of the study, including the maintenance of scientific and ethical standards, the well-being of CHILD participants, and the overall co-ordination of studies and personnel. She also leads the in-depth evaluation of lung function trajectories from infancy through childhood undertaken at the Toronto site. |
|
Meghan Azad, PhD
IMiC Scientific Director; Deputy Director, CHILD Cohort Study Dr. Azad is a Professor of Pediatrics and Child Health at the University of Manitoba and holds a Tier 2 Canada Research Chair in Developmental Origins of Chronic Disease. Her research program is focused on the role of infant nutrition and the microbiome in child growth, development and resilience. Dr. Azad is leading a clinical trial to improve matching procedures for preterm neonates receiving donor human milk, and directs the new International Milk Composition (IMiC) Consortium. Dr. Azad also serves as Secretary for The International Society for Research in Human Milk and Lactation (ISRHML) and as an Expert Member on the NIH Breastmilk Ecology: Genesis of Infant Nutrition (BEGIN) Working Group. |
|
ELICIT contributes milk samples to IMiC for various analyses. These samples were collected from participants in an RCT performed in the area around Haydom, Tanzania— a rural, maize-based agricultural setting with a high burden of intestinal pathogens and high rate of childhood stunting. The factorial RCT ultimately demonstrated that scheduled antimicrobials and/or daily nicotinamide did not improve childhood growth for 1188 children recruited at age <2 weeks and followed until 18 months — though the rich detail of assessments permits better understanding of contributors to poor growth.
|
|
Mark DeBoer, MD, MSc, MCR | UVa Principal Investigator
Pediatric Endocrinologist, University of Virginia (UVa) As a pediatric endocrinologist, Dr. Mark D. DeBoer is passionate about learning how we can improve childhood growth and development in resource-poor settings around the world. He is the UVa PI of ELICIT and other studies in the area around Haydom Tanzania. He is also active in research related to metabolic syndrome and the use of diabetes technology in the care of children with T1D. |
|
Joann M. McDermid, MSc, DipEpidBiostat, PhD, RDN, FAND | Co-Investigator
Consultant Dr. McDermid is a Registered Dietitian Nutritionist who completed her PhD in Epidemiology & Population Health at the London School of Hygiene and Tropical Medicine in the United Kingdom. Dr. McDermid is interested in maternal and infant nutrition and in the complex interactions between dietary intake, nutritional health and autoimmune or infectious disease outcomes in domestic and international contexts. |
|
Estomih Mduma, MPH | ELICIT Principal Investigator
Research Consultant, Haydom Global Health Research Center Estomih Mduma is a researcher in epidemiology and clinical trials research, leading a large research team at the Haydom Global Health Research Center. He is Principle Investigator of multiple projects, including ELICIT and MAL-ED. He has an extensive background in research on improving neonatal resuscitation techniques through the SAFER Birth study. |
|
The MISAME-3 (MIcronutriments pour la SAnté de la Mère et de l’Enfant) research project aims to study the efficacy of a fortified balanced energy protein (BEP) supplement for pregnant and lactating women to improve birth weight, fetal and infant growth in rural Burkina Faso. MISAME-3 contributes milk samples to IMiC for various analyses.
Website: www.misame3.ugent.be |
|
Patrick Kolsteren, PhD
Professor, Food Technology, Safety and Health, Ghent University Patrick Kolsteren is a Professor at the Department of Food Technology, Safety and Health at Ghent University Belgium. He works on child health and nutrition, with a focus on evidence-based medicine. He is currently Chair of International Nutrition at Ghent University and has conducted randomized clinical trials and studies in Asia, Africa and Latin America. He has consulted for IFAD, WHO, FAO, UNICEF, the Belgian and German Development Cooperation and many NGOs. |
Additional Team Member:
- Trenton Dailey-Chwalibóg, PhD
|
A community-based, randomized control, assessor blinded trial in peri-urban settings of Karachi, Pakistan to study the impact of Lipid-based Nutritional Supplement for Pregnant and Lactating women which is balanced energy-protein (BEP) dietary supplement, a locally produced ready-to-use nutritional product for lactating women (LW) and single prophylaxis dose of Azithromycin for infants, on growth of infants over the period of six months since birth compared to current standard of care. VITAL contributes human milk samples to IMiC for various analyses.
Website: www.vitalpakistan.org.pk |
|
Yasir Shafiq, BPharm, MSc | Principal Investigator
Senior Manager, VITAL Pakistan; Research Specialist, The Aga Khan University Yasir oversees management of VITAL’s core program & leads their research initiatives around nutrition. He is also responsible for scaling up VITAL’s routine immunization program to different communities in Karachi. Yasir is a member of the GAVI CSO steering committee; he has over 10 years implementation experience on MNCH interventions in resource contraints settings. |
|
Fyezah Jehan, MSc, FCPS, MBBS | Co-Investigator
Associate Professor & Chairperson, Pediatrics and Child Health, The Aga Khan University Fyezah is the technical lead at AKU for the design, oversight, and analysis of the MUMTA trials and provides laboratory services (blood/urine/stool testing) for pregnant women and infants and MRI support. |
Additional Team Members:
- Ameer Muhammad, MS, Pharm-D
- Imran Nisar
Laboratory Partners
|
Human Milk Oligosaccharides (HMOs) are the third most abundant component of human breast milk. The goal of our research is to reveal which maternal factors influence HMO composition and discover how HMOs impact health and development. The Bode Lab in collaboration with the Mother-Milk-Infant Center of Research Excellence (MOMI CORE) performs HMO & Targeted Protein analysis on IMiC human milk samples.
Websites: www.bodelab.com / www.milk.ucsd.edu |
|
Lars Bode, PhD
Professor of Pediatrics, UCSD; Director, MOMI CORE Dr. Bode has been working on human milk oligosaccharides (HMOs) for over 20 years. Dr. Bode is Professor of Pediatrics in the Division of Neonatology and the Division of Gastroenterology, Hepatology and Nutrition, the Larsson-Rosenquist Chair of Collaborative Human Milk Research, and Director of the Larsson-Rosenquist Foundation Mother-Milk-Infant Center of Research Excellence (MOMI CORE) at the University of California, San Diego (UCSD). |
Additional Team Members:
- Annalee Furst
- Kristija (Kristy) Sejane
- Nikhila (Nikki) Yerabandi
- Chloe Sermet
|
The Maternal, Infant and Lactation Quality (MILQ) Study, led by Dr. Lindsay Allen, is collecting milk, blood, saliva and fecal samples across the first nine months of lactation, in well-nourished women in four countries. Dr. Allen's laboratory team analyzes micronutrients and other constituents of IMiC human milk samples using platforms such as UPLC-MS/MS, in non-intervention and intervention studies.
Website: Allen (MILQ) Lab - USDA ARS |
|
Lindsay Allen, PhD
Research Physiologist, USDA, ARS Western Human Nutrition Research Center Principal Investigator, MILQ Study; Research Professor, UC Davis Dr. Lindsay Allen is a Research Physiologist in the USDA, ARS Western Human Nutrition Research Center in Davis, CA, where until recently she was the Center Director. She studies prevalence, causes and consequences of micronutrient deficiencies using randomized, controlled human trials testing micronutrient supplements, food fortification, and food-based approaches to improve nutritional status, pregnancy outcome and child development. Dr. Allen served on committees of the Food and Nutrition Board, WHO, UNICEF, ADB, World Bank, PAHO and FAO and was principal author of WHO/FAO’s “Guidelines on Food Fortification with Micronutrients”. |
|
Daniela Hampel, PhD
Food Chemist, MILQ Study; Associate Project Scientist, UC Davis USDA, ARS Western Human Nutrition Research Center Daniela is a Food Chemist and the Analytical Coordinator of the MILQ Study at the USDA/ARS Western Human Nutrition Research Center and the Department of Nutrition at the University of California Davis. Her research has encompassed three areas: food chemistry, molecular biology, and nutrition, and included method development and studies on natural compounds with potential health benefits in each area. Within the field of nutrition, her focus is method development for nutrient analyses in human milk and plasma/red blood cells to assess micronutrient deficiencies in mostly low- and middle-income countries. |
Additional Team Member:
- Setareh Shahab-Ferdows | Nutritionist, Lab Manager
|
Cedars-Sinai Precision Biomarker Laboratories (PBL) is a proteomic biomarker discovery and development services provider that aims to leverage proteomics to realize the potential of precision medicine. Our experts enable precise proteome phenotyping of a wide range of body fluids, cells and tissues by integrating novel proteomic methods and translational capabilities to uncover and quantify clinically useful biological and pathological signatures. In collaboration with the Manitoba Interdisciplinary Lactation Centre (MILC) research consortium, PBL has established and optimized a high throughput proteomics workflow for human breast milk in order to quantify the biological composition across population-based cohorts.
Website: www.cedars-sinai.org/pbl |
|
Jennifer Van Eyk, PhD
Jennifer Van Eyk, PhD, is an international leader in the area of clinical proteomics and her lab has focused on developing technical pipelines for de novo discovery and larger scale quantitative mass spectrometry methods. This includes multiple reaction monitoring (MRM, also known as SRM) and most recently data independent acquisition. Dr. Van Eyk's laboratory is well known for the extreme technical quality of the data generated, rigorous quality control with tight %CV while applying these to key clinical questions. The aim is to maximize throughput and reproducibility in order to move targeted and robust discovery methods into large population healthy continuous assessment and clinical grade assays focusing on brain and cardiovascular diseases. |
|
Sapient is a full-service bioanalytical research organization that enables rapid discovery of circulating biomarkers by combining high throughput mass spectrometry technologies, biocomputational learning, and proprietary longitudinal human data. These tools and methods quickly deliver actionable insights into biomarkers of human health, biological processes, pharmacodynamics, and disease progression. Sapient performs untargeted biomarker discovery and analysis on IMiC milk samples from our Field Site Partners.
Website: https://sapient.bio/ |
|
Julia Gauglitz, PhD
Principal Investigator, Sapient Dr. Gauglitz is an analytical chemist with deep expertise in small molecule mass spectrometry and its application in chemical analysis. Prior to joining Sapient, she performed her postdoctoral scientific training at Woods Hole Oceanographic Institution under a National Science Foundation (NSF) postdoctoral research award, and subsequently at the University of California, San Diego. There she founded and served as leader of the Global FoodOmics project to map nutritional chemistry and microbiology on a global scale, and enabled tool development for investigating untargeted mass spectrometry data. At Sapient, Dr. Gauglitz oversees key customer relationships and serves as scientific liaison for clients. |
|
Lori Glenwinkel, PhD
Biological Data Scientist, Sapient Dr. Glenwinkel is an experienced computational and molecular biologist with a strong background in the field of molecular genetics and development. Her previous research has involved analyzing large genomics datasets for the identification of causal variants as well as single cell RNA sequencing data processing and visualization, and has informed her approach to answering scientific questions at both the cellular and population levels. At Sapient, one of her primary focus areas is in the identification of biomarkers related to maternal and infant health. |
|
Mo Jain, MD, PhD
Founder and CEO, Sapient Dr. Jain is a physician-scientist with nearly 20 years of expertise in physiology, biomedicine, engineering, computational biology, and mass spectrometry-based metabolomics. Prior to founding Sapient, he formed and was director of Jain Laboratory at the University of California, San Diego (UCSD), which focused on developing next-generation mass spectrometry-based systems to probe the non-genetic landscape of disease across population-scale human studies. Dr. Jain founded Sapient to expand upon this mission of accelerating human discovery through the nexus of high throughput analytical mass spectrometry, computational biology, and population-scale clinical studies. |
Additional Team Members:
- Han Steger, PhD | Postdoctoral Researcher
- Christopher Cheng | Project Manager
- Tao Long, PhD, MBA | Head of Data Sciences
- Zi Ye | Biological Data Scientist
- Han Steger, PhD | Postdoctoral Researcher
- Jeramie Watrous, PhD | Head of Analytical R&D

Biocrates Life Sciences
Innsbruck, Austria
Innsbruck, Austria
|
Biocrates Life Sciences is the global leader in mass spectrometry-based quantitative metabolite technology. Its kits and services cover over 1,000 metabolites from more than 40 metabolite classes.
Biocrates' metabolomics profiling products provide quantitative, reproducible assays designed in a scalable and easy-to-use kit format. This format combined with Biocrates' expertise in study design, sampling strategies, sample measurement, statistical analysis, and biomedical data interpretation, reveals the driving factors behind biological differences and provides insights into underlying mechanisms. Biocrates performs comprehensive metabolomics analysis on IMiC milk samples from field site partners, providing quantitative metabolite data to the project. Website: https://biocrates.com/ |
|
Gordian Adam, PhD
Team Lead of Data Interpretation, biocrates life sciences Dr. Gordian Adam is a senior biochemist with extensive expertise in metabolomics data analysis, biological meaning, and medical relevance of alterations in biochemical pathways. At biocrates, Dr. Adam leads a team of skilled scientists experienced in statistical analysis and data interpretation. Before focusing on metabolomics, he conducted research in oncology and immuno-oncology at Bayer Pharmaceuticals, Germany, and Immunocore Ltd., UK. Within metabolomics, he has experience in extraction of crucial findings from large and complex datasets to identify biomarkers and potential drug targets based on metabolic and systems biology data. Dr. Adam contributes to the IMiC team through metabolomics data compilation, normalization, and quality control. |
|
Marissa A. Jones, PhD
Business Development Manager, biocrates life sciences Dr. Jones is an analytical chemist with expertise in mass spectrometry and metabolomics. Before joining biocrates, Dr. Jones earned her PhD in analytical chemistry at Vanderbilt University in Prof. Richard Caprioli's lab. At biocrates, Dr. Jones seeks to make metabolomics accessible to everyone by leveraging her training as an analytical chemist and her experience as a business development manager to support scientists in advancing their projects both within and outside of biocrates life sciences. Dr. Jones contributes to the IMiC team by serving as the scientific liaison between biocrates and the consortium. |
|
Fadi Abdi, PhD
Chief Commercial Officer, biocrates life sciences Dr. Abdi is responsible for biocrates' strategic partnership building to scale metabolomics for large consortia like IMiC. Furthermore, he and his team shape biocrates' product commercialization and grow the company's footprint in the biomedical science community. Prior to joining biocrates in 2018, Dr. Abdi applied his skills in genomics, proteomics, and metabiolomics in different leadership positions at Biognosys and SCIEX. |
Additional Team Members:
- Denise Sonntag, PhD | Project management
- Cornelia Rohring, PhD | Head of Analytical Services
Data Science Partners
|
The Aghaeepour Lab uses machine learning to study patients in translational settings. This includes integrative “multiomics” analysis across genomics, proteomics, imaging, and single-cell technologies, as well as quantitative clinical phenotyping using electronic health records and wearable devices.
Website: https://nalab.stanford.edu/ |
|
Nima Aghaeepour, PhD
Assistant Professor, Stanford University Nima Aghaeepour is an Assistant Professor at Stanford University. His laboratory develops machine learning and artificial intelligence methods to study clinical and biological modalities in translational settings. He is primarily interested in leveraging multiomics studies and wearable devices to address global health challenges. His work is recognized by awards from numerous national and international organizations including the Bill and Melinda Gates Foundation, the March of Dimes Foundation, the Burroughs Wellcome Fund, the National Institute of General Medical Sciences, and the National Center for Advancing Translational Sciences. |
Additional Team Member:
- Martin Becker, PhD
More Data Science Partner Profiles Coming Soon
Hubbard Group:
IMiC Database:
Ki Data Services:
Hubbard Group:
- Alan Hubbard
IMiC Database:
- Krista Pace
- Justin Foong
Ki Data Services:
- Vishak Subramoney
|
The IMiC Consortium is funded by a US$5,000,000 investment from the Bill & Melinda Gates Foundation Maternal, Newborn & Child Health Strategy.
|